9/22/2014 U.S. and Canadian regulators have green lighted the use of its treatment 9.22.2014
September 22, 2014 / SEP/22/2014
NEW YORK (TheStreet) -- Tekmira Pharmaceuticals (TKMR_) shares are up 12.8% to $22.74 after the announced that U.S. and Canadian regulators have green lighted the use of its treatment, TKM-Ebola, for patients with confirmed or suspected cases of Ebola.
The company said that its treatment has already been used in emergency cases and has been generally been well tolerated by patients.
The company is developing TKM-Ebola in conjunction with the U.S. Defense Department.
Must Read: Warren Buffett's 25 Favorite StocksSTOCKS TO BUY: TheStreet Quant Ratings has identified a handful of stocks that can potentially TRIPLE in the next 12 months. Learn more.
"Tekmira is reporting that an appropriate regulatory and clinical framework is now in place to allow the use of TKM-Ebola in patients. We have worked with the FDA and Health Canada to establish this framework and a treatment protocol allowing us to do what we can to help these patients," said Tekmira's President and CEO Dr. Mark J. Murray.
TheStreet Ratings team rates TEKMIRA PHARMACEUTICALS CORP as a Sell with a ratings score of D. TheStreet Ratings Team has this to say about their recommendation:
"We rate TEKMIRA PHARMACEUTICALS CORP (TKMR) a SELL. This is driven by some concerns, which we believe should have a greater impact than any strengths, and could make it more difficult for investors to achieve positive results compared to most of the stocks we cover. The company's weaknesses can be seen in multiple areas, such as its feeble growth in its earnings per share, deteriorating net income, disappointing return on equity and weak operating cash flow."
Highlights from the analysis by TheStreet Ratings Team goes as follows:
The company said that its treatment has already been used in emergency cases and has been generally been well tolerated by patients.
The company is developing TKM-Ebola in conjunction with the U.S. Defense Department.
Must Read: Warren Buffett's 25 Favorite StocksSTOCKS TO BUY: TheStreet Quant Ratings has identified a handful of stocks that can potentially TRIPLE in the next 12 months. Learn more.
"Tekmira is reporting that an appropriate regulatory and clinical framework is now in place to allow the use of TKM-Ebola in patients. We have worked with the FDA and Health Canada to establish this framework and a treatment protocol allowing us to do what we can to help these patients," said Tekmira's President and CEO Dr. Mark J. Murray.
TheStreet Ratings team rates TEKMIRA PHARMACEUTICALS CORP as a Sell with a ratings score of D. TheStreet Ratings Team has this to say about their recommendation:
"We rate TEKMIRA PHARMACEUTICALS CORP (TKMR) a SELL. This is driven by some concerns, which we believe should have a greater impact than any strengths, and could make it more difficult for investors to achieve positive results compared to most of the stocks we cover. The company's weaknesses can be seen in multiple areas, such as its feeble growth in its earnings per share, deteriorating net income, disappointing return on equity and weak operating cash flow."
Highlights from the analysis by TheStreet Ratings Team goes as follows:
- TEKMIRA PHARMACEUTICALS CORP's earnings per share declined by 27.3% in the most recent quarter compared to the same quarter a year ago. The company has reported a trend of declining earnings per share over the past two years. During the past fiscal year, TEKMIRA PHARMACEUTICALS CORP swung to a loss, reporting -$0.96 versus $1.87 in the prior year.
- The company, on the basis of change in net income from the same quarter one year ago, has significantly underperformed when compared to that of the S&P 500 and the Biotechnology industry. The net income has significantly decreased by 95.8% when compared to the same quarter one year ago, falling from -$3.11 million to -$6.08 million.
- Return on equity has greatly decreased when compared to its ROE from the same quarter one year prior. This is a signal of major weakness within the corporation. Compared to other companies in the Biotechnology industry and the overall market, TEKMIRA PHARMACEUTICALS CORP's return on equity significantly trails that of both the industry average and the S&P 500.
- Net operating cash flow has significantly decreased to -$8.01 million or 173.84% when compared to the same quarter last year. In addition, when comparing to the industry average, the firm's growth rate is much lower.
- The revenue fell significantly faster than the industry average of 43.1%. Since the same quarter one year prior, revenues fell by 38.2%. The declining revenue appears to have seeped down to the company's bottom line, decreasing earnings per share.
- You can view the full analysis from the report here: TKMR Ratings Report
What are you think on Tekmira? Is that: Much More Than An Ebola Treatment?
Tekmira Pharmaceuticals Corporation (NASDAQ:TKMR) has been in the news recently as one of the leaders in developing a treatment for the Ebola Virus. It is a Vancouver based, publicly traded company.
Because of the recent Ebola outbreak, Tekmira’s stock has fluctuated wildly. No one can predict what will occur in this sort of epidemic and similarly no one can accurately predict the short term course of their stock.
However, Tekmira has fascinating and leading edge technology that should make it a major player in a raft of diseases. The long term success of this company may be tremendous.
I will first discuss the role it is currently playing in the Ebola outbreak. Then, I will go on to explain the meat of this impressive company.
Tekmira: Rating the Company
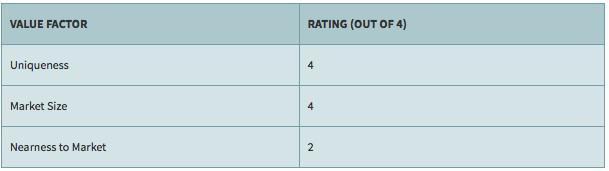
Tekmira is one of the leading exponents of Nobel Prize winning technology. That alone, would make it an interesting company.
Tekmira has also developed the top delivery system in the world to deliver this technology to appropriate sites in patients. Accordingly, other leading exponents of this technology will likely be seeking partnerships with Tekmira.
Tekmira and the Ebola Virus
Much has been written about the current outbreak in Africa of Ebola. As everyone knows by now, it is extremely virulent with a 50-90% mortality rate. There is no known cure. Furthermore, medications to try to treat Ebola have not yet been subjected to rigorous testing.
This leads to a significant political conundrum. To not treat Africans with anti Ebola treatment, while 2 Americans were successfully treated with an experimental drug would be viewed, at the very best, as elitist. To treat hundreds of third world patients with untested drugs could be viewed as tantamount to using them as guinea pigs.
The World Health Organization finally decided to go ahead with treatment. The two leading research drugs are ZMAPP, manufactured in the US and Tekmira’s TKM-Ebola.
ZMAPP was used successfully on two Americans and unsuccessfully on a Spaniard. At this point we don’t really know whether the two patients survived because of the medication or because of good supportive care in an excellent hospital.
Tekmira’s product has yet to be used. It should be noted that the two products work entirely differently. Most experts feel that TKM-Ebola is further advanced in testing. That, of course, does not mean that it is a superior product. In fact Tekmira put a small human study on hold due to safety concerns (which have been at least partly alleviated). The US government prior to this outbreak decided to fund Tekmira’s research to the tune of $140 Million.
ZMAPP is in such short supply that stocks of the drug are already depleted, TKM-Ebola is available in only slightly greater amounts. The fact is, neither company can meet the short term need and both companies will take at least 3 months to supply more medication.
Tekmira and RNA Interference
In 2006 Andrew Fire and Craig C. Mello won the Nobel Prize for Physiology and Medicine for their work on RNA interference.
Proteins control most processes in the body. Protein signaling is crucial to many normal and pathologic processes. The production of these proteins is controlled mostly by RNA. More specifically messenger RNA (mRNA) lays down the template to produce proteins.
What happens when a protein is instrumental in causing a disease? It turns out that a natural mechanism of the body is to try to splice in a small fragment of RNA into the crucial piece of mRNA that produces the protein in question. It makes sense as a defense mechanism. Unfortunately, it often doesn’t work and the disease progresses.
The discovery of this naturally occurring mechanism is what won Fire and Mello the Nobel Prize. They discovered two types of small bits of RNA that could interfere with specific protein formation, known as micro RNA and small interfering RNA (siRNA). At the time, the discovery was of tremendous theoretical value but little practical use.
Times have changed. Biochemists can now design small bits of RNA. Scientists can specifically splice in bits of RNA exactly where they want to. Furthermore, the human genome project mapped out our entire protein producing code. Now specific targets can be identified and their functions can be turned up or more easily, turned down by siRNA.
In theory, thousands of diseases, from viruses to metabolic abnormalities will soon be cured by siRNA, spliced into appropriate positions. Tekmira is one of the world leaders in research in this field.
Tekmira and its LNP Delivery Platform
Why haven’t most of the world’s diseases been solved then? First and foremost is the fact that most diseases don’t have a readily identifiable protein as their cause. The number that do will increase markedly in the next few years.
The second reason is that, as with many new treatments, delivery to the appropriate site is an issue.
Tekmira, as it turns out, is not only a world leader in siRNA research, but also the world leader in the delivery of these tiny bits of RNA. Their system is called Lipid Nanoparticle Technology (LNP). A third generation LNP is in use and a fourth generation is being developed. The implication of this is that other world leaders in siRNA who want to optimize their delivery may have to partner with Tekmira.
What are the pluses of LNP? It is designed to stay in circulation for a long time.
- Distribution of the siRNA is rapid and efficient.
- It concentrates at the site of malignancy.
- siRNA is only released in the cell, not in circulation.
- Because of lack of extracellular release it minimizes immune toxicity.
- Potency has increased.
- Lyophilization, a freeze drying process for easy storage and transport, has been developed.
- Potency is maintained long term (prior to administration).
- The drugs can be given subcutaneously.
- Respiratory administration is available.
Tekmira’s Pipeline
I’m only going to briefly discuss Tekmira’s pipeline because it’s really the long term potential of the marriage of siRNA technology and LNP technology that is important. What we are seeing now is the tip of the proverbial iceberg.The first product in the pipeline is TKM-PLK1. This represents Tekmira’s Oncology candidate. Tumors that produce a lot of PLK protein have extremely poor prognosis. Splicing in appropriate siRNA and delivering it via LNP turned out to be relatively easy.
When PLK1 expression is inhibited, the cell cycle is completely arrested and cell division is impossible. Obviously, this is a useful concept for cancer therapy. Phase 1 results were good and Tekmira is currently conducting Phase 1/Phase 2 trials for safety, tolerability and pharmacokinetics on 2 rare types of cancer and on hepatocellular carcinoma (primary liver cancer) which is common.
The second group of products in the pipeline is anti-virals. TKM-Ebola is in Phase 1 studies. Currently all product is being sent to Africa and the study will resume after the crisis is over.
Perhaps the most interesting paper on siRNA and LNP delivery on Ebola was the publication in conjunction with Boston University and the U.S. Army Medical Research Institution.
In this study they took non human primates previously infected with Ebola. The animals were injected with a lethal dose of Ebola virus and with TKM Ebola. All of the animals survived. This was a great part of the impetus for the American government to give a $140 Million grant to Tekmira, which funded basically all the improvements to the LNP system.
TKM-HBV is a product designed to treat Hepatitis B Virus (HBV). Many other small molecules have had success treating Hep B but fail to eliminate the HBV surface antigen. This antigen (a protein) leads to chronic inflammation which in turn can cause non-alcoholic cirrhosis and sometimes liver cancer. TKM-HBV targets multiple sites on the genome and eliminates the surface antigen.
Current treatments are prohibitively expensive ( the most effective treatment is in the $100,000 range).
There is also a product in development — TKM-Marburg — to treat the deadly Marburg virus which is quite similar to Ebola.
Pathologic proteins can cause inherited metabolic disorders. Drugs are in development for rare diseases causing high triglycerides and for disordered storage of glycogen.
Another medication in the pipeline is TKM-ALDH2. This product inhibits Aldehyde Dehydrogenase Enzyme 2 (a protein). In doing so, it causes a build up of Acetaldehyde. People with a build up of Acetaldehyde get nasty reactions when they drink alcohol. The reactions are uncomfortable enough that these people stop ingesting alcohol.
The product achieves the same effect as Antabuse. Antabuse has to be taken each day and has poor compliance amongst alcoholics. TKM-ALDH2 lasts weeks.
Tekmira Partnerships
Other researchers with siRNA technology are partnering with Tekmira for The LNP delivery technology. Foremost amongst them is Alnylam (NASDAQ:ALNY). Alnylam has developed three treatments in conjunction with Tekmira. Currently the lead compound is ALN-TTRO2 which has had a very successful Phase 2 trial, treating amyloidosis and is starting a Phase 3 trial. This has triggered a $5 Million milestone payment to Tekmira.Tekmira: What Lies Ahead?
As stated in the introduction there really is no way to predict the short term movement of this company’s stock. With the current Ebola problem, the stock has gone up as much as 60% in one day and plummeted as much as 41% in two days. Certainly Tekmira can sell their Ebola treatment as fast as they can make it.The future of Tekmira will not depend on selling drugs to combat Ebola. Medication of this type is given on a compassionate basis and garners little revenue. It’s also far too early to know if this drug is a long term viable option to treat Ebola.
Long term, I like what this company is doing. They are a world leader in a Nobel Prize winning technology and have developed the best delivery system for that technology. So far, that technology is only being applied to a few diseases. How successful this company becomes will depend on identifying more and more proteins to target.
I’m optimistic. I believe that the vast fields of virology and oncology await (as well as other fields). I’m not alone in my optimism. Doug Loe, an analyst with Euro Pacific Canada sees “upside potential” due to a “wider array” (not just Ebola treatment) in the pipeline. He refers to Tekmira as a good speculative buy.
Adrian Mantraca with KCM Wealth Management considers Tekmira an “aggressive investment”.
This company may be poised to do big things but despite the recent stock fluctuations I think that any great success is still quite far off.
Disclosure: The author has no positions in any of the stocks mentioned. The author wrote this article themselves and is not receiving compensation for it.
Posted at:
1) http://markman.ca/2014/08/tekmira-much-more-than-an-ebola-treatment/
2) http://seekingalpha.com/article/2493765-tekmira-much-more-than-an-ebola-treatment
3) That is really important to many of incestors, please share your comments
US STOCKS-Tekmira Pharma, AbbVie among premarket actives
By Chuck Mikolajczak
NEW YORK, Sept 23 (Reuters) - U.S.-listed shares of Tekmira Pharmaceuticals were climbing on Tuesday, up 8.9 percent at $25.70 as one of the most actively trades Nasdaq stocks as its Ebola drug will be one of several to be tested in West Africa in a bid to win fast-track trials.
Volume of nearly 170,000 places the stock among the top three most actively traded shares on the Nasdaq before the opening bell and represents about 5 percent of its 10-day average.
Tekmira shares rose more than 17 percent on Monday after U.S. and Canadian regulators authorized the use of its Ebola treatment in patients who have confirmed or suspected infections from the deadly virus.
Shares of AbbVie were down 4.6 percent at $55.99 in premarket trading after the Obama administration took several actions late Monday that will reduce the tax benefits available to companies that have done inversion deals, while also making new inversions more difficult to do and potentially less rewarding.
Consolidated volume of nearly 400,000 made AbbVie the most actively traded issue on the New York Stock Exchange and represented about 3.1 percent of its 10-day average.
AbbVie has agreed to a deal to acquire Shire, which fell 5.5 percent and was also among the most actively traded Nasdaq names.
U.S. stock index futures were moving lower on Tuesday, putting the S&P on track for a third straight decline, as conflict in the Middle East intensified and after the U.S. Treasury moved to curb "tax inversion" deals.
The United States and Arab allies bombed Syria for the first time on Tuesday, killing dozens of Islamic State fighters and members of a separate al Qaeda-linked group, pursuing a campaign against militants into a war at the heart of the Middle East.
Data expected on Tuesday includes the flash September reading on manufacturing from financial data firm Markit at 9:45 a.m. (1345 GMT). Expectations call for a reading in the main purchasing managers' index of 58 versus the final 57.9 in August.
Salix Pharmaceuticals was trading up 9.3 percent at $174.76 in the premarket after a person familiar with the matter said on Monday that Allergan Inc had revived discussions to buy the company.
Norway's Yara and Chicago-based CF Industries are in talks about a merger of equals that could create a $27 billion global fertilizer producer, rivaling Canada's Potash Corp in size in a fragmented and oversupplied market.
* S&P 500 e-minis were falling 6 points, or 0.3 percent, with 174,767 contracts changing hands.
* Nasdaq 100 e-minis were down 14.5 points, or 0.36 percent, in volume of 28,821 contracts.
* Dow e-minis were down 44 points, or 0.26 percent, with 24,277 contracts changing hands.
(Reporting by Chuck Mikolajczak; Editing by Lisa Von Ahn)
Read more: http://www.dailymail.co.uk/wires/reuters/article-2766544/US-STOCKS-Tekmira-Pharma-AbbVie-premarket-actives.html#ixzz3EjJyJCHC
Follow us: @MailOnline on Twitter | DailyMail on Facebook
No comments:
Post a Comment